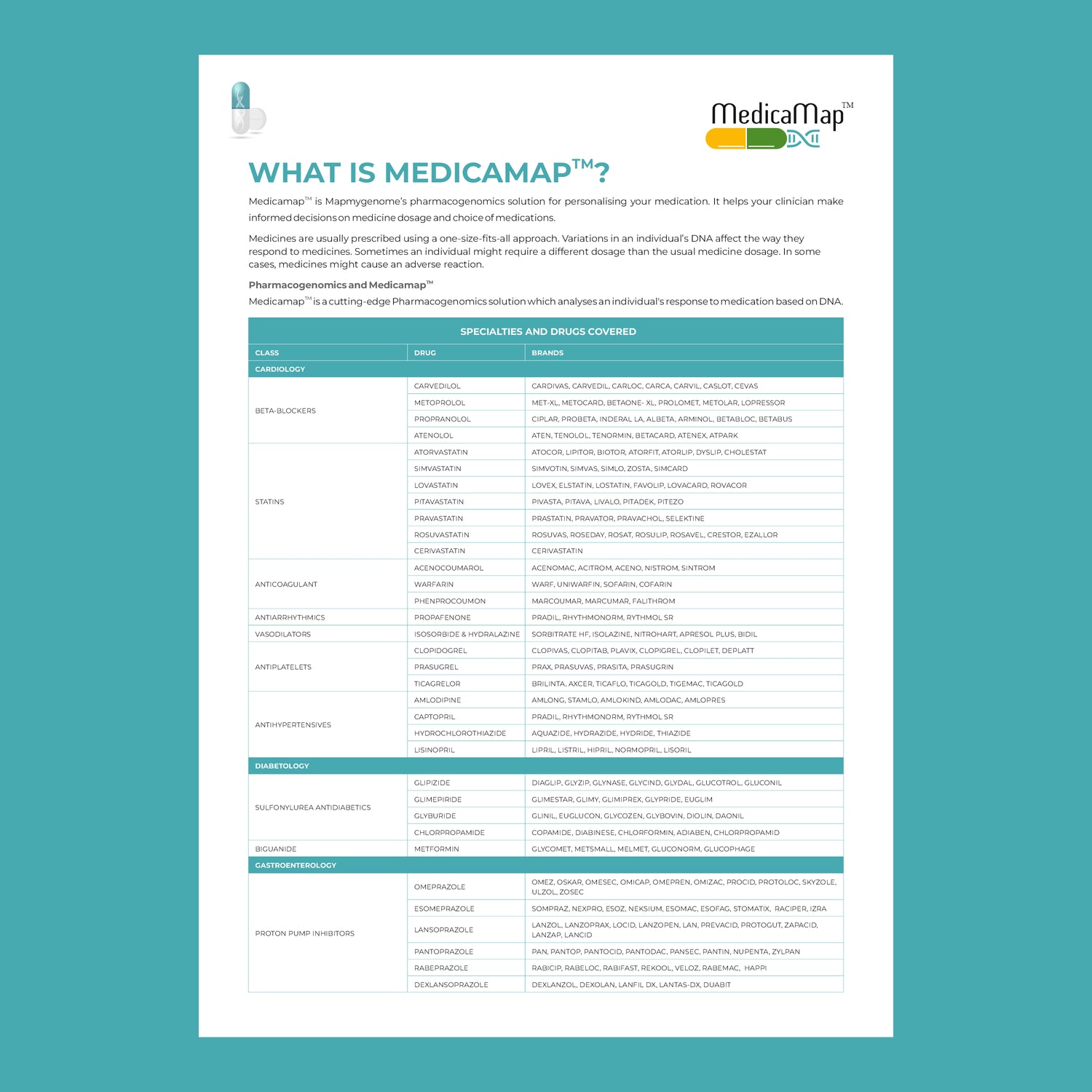
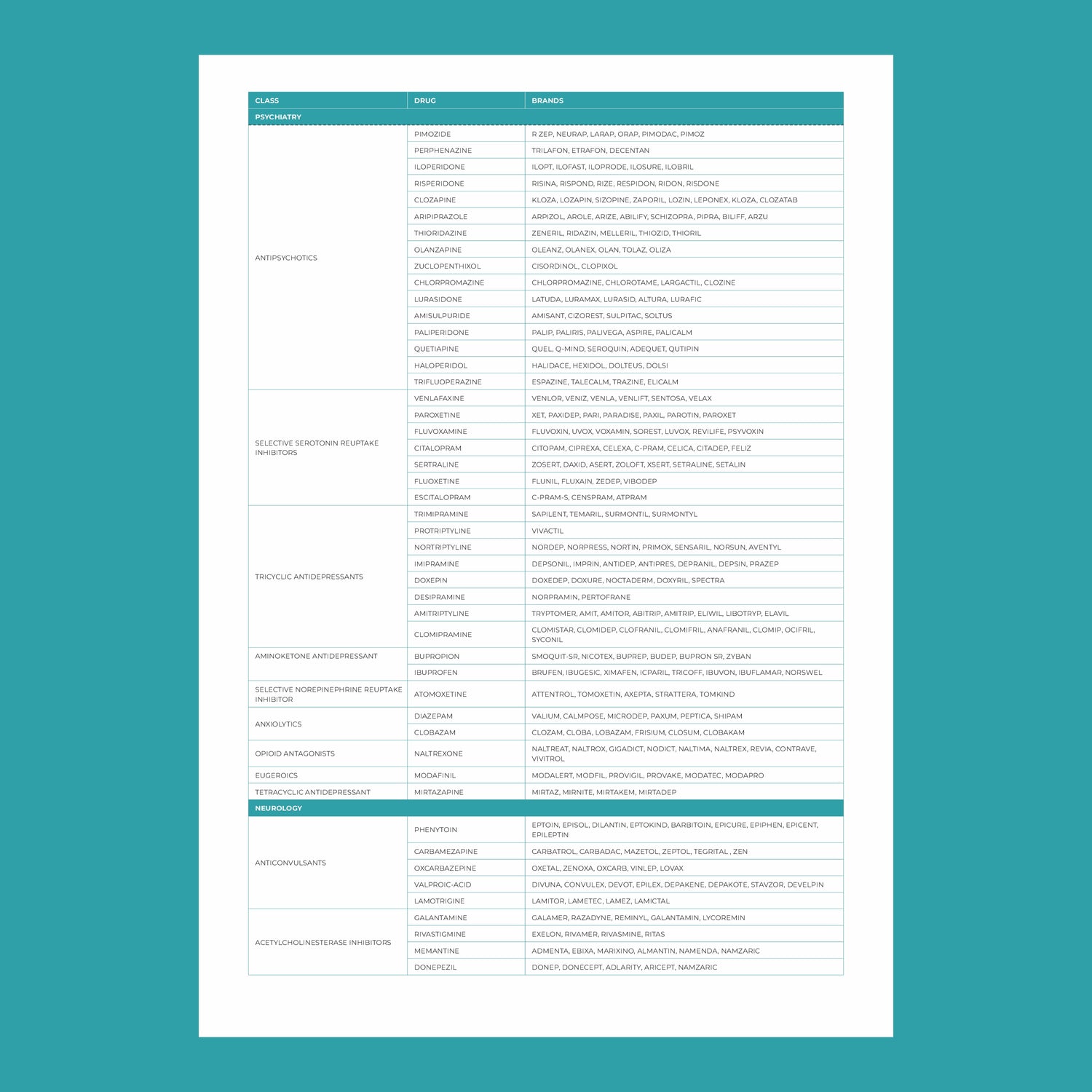
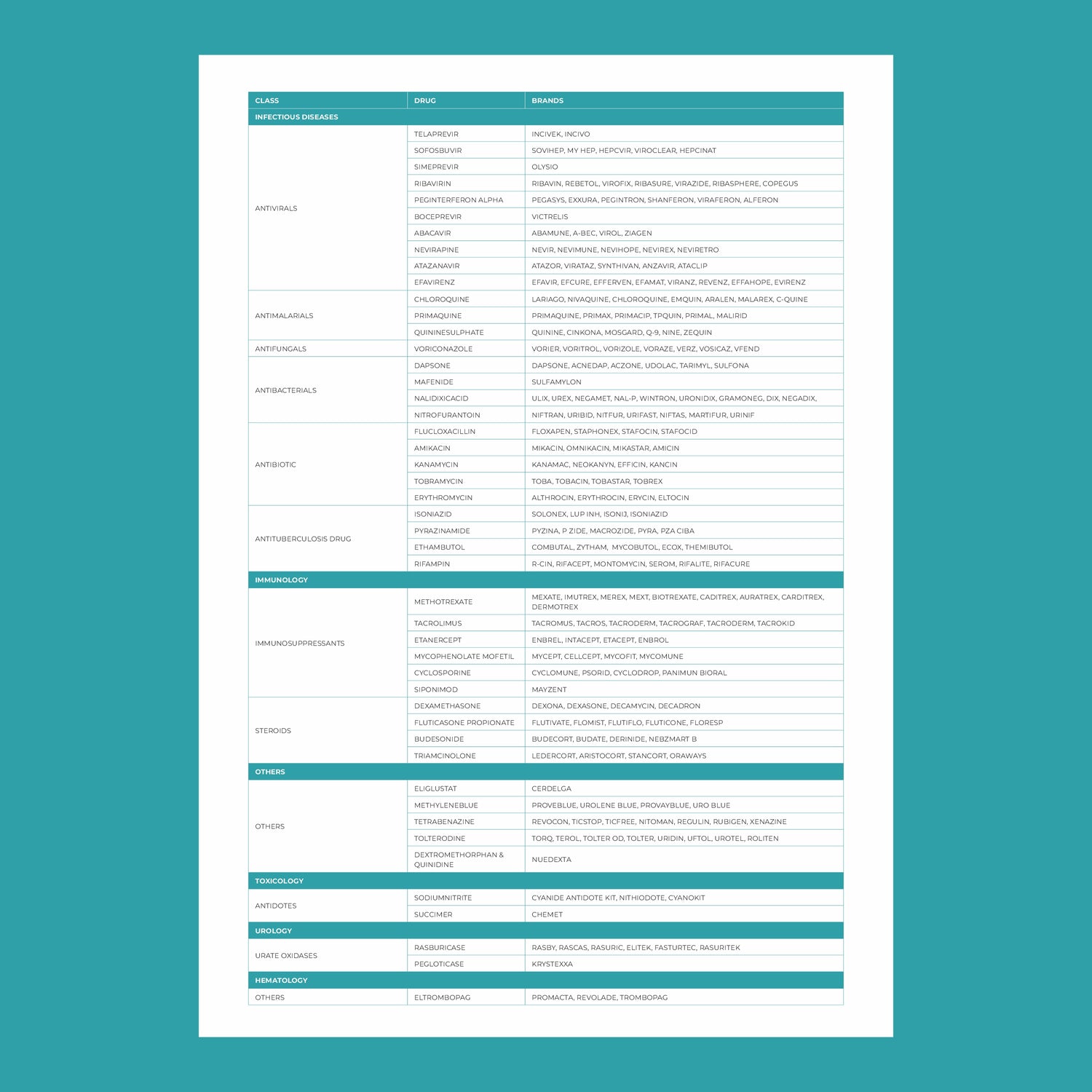
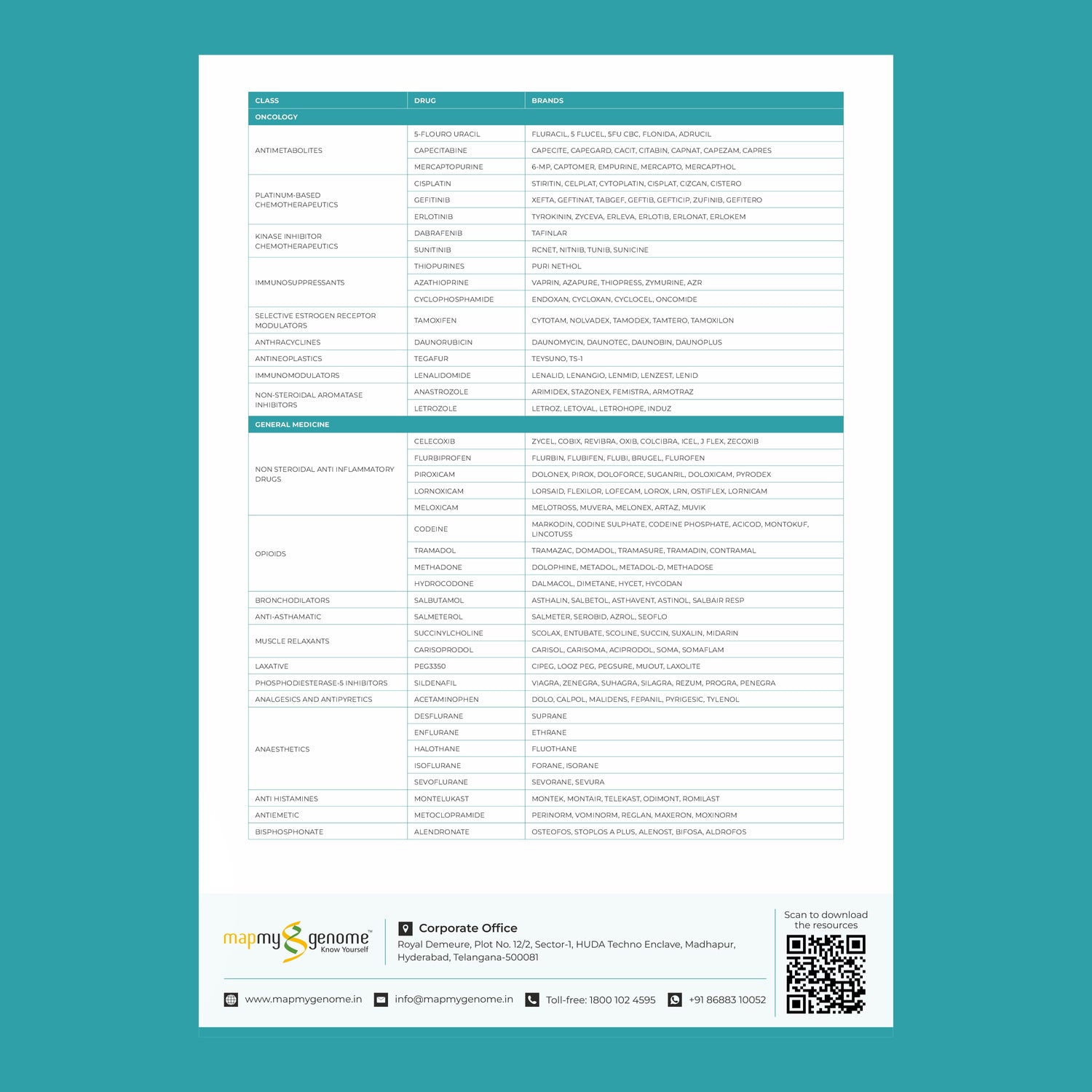
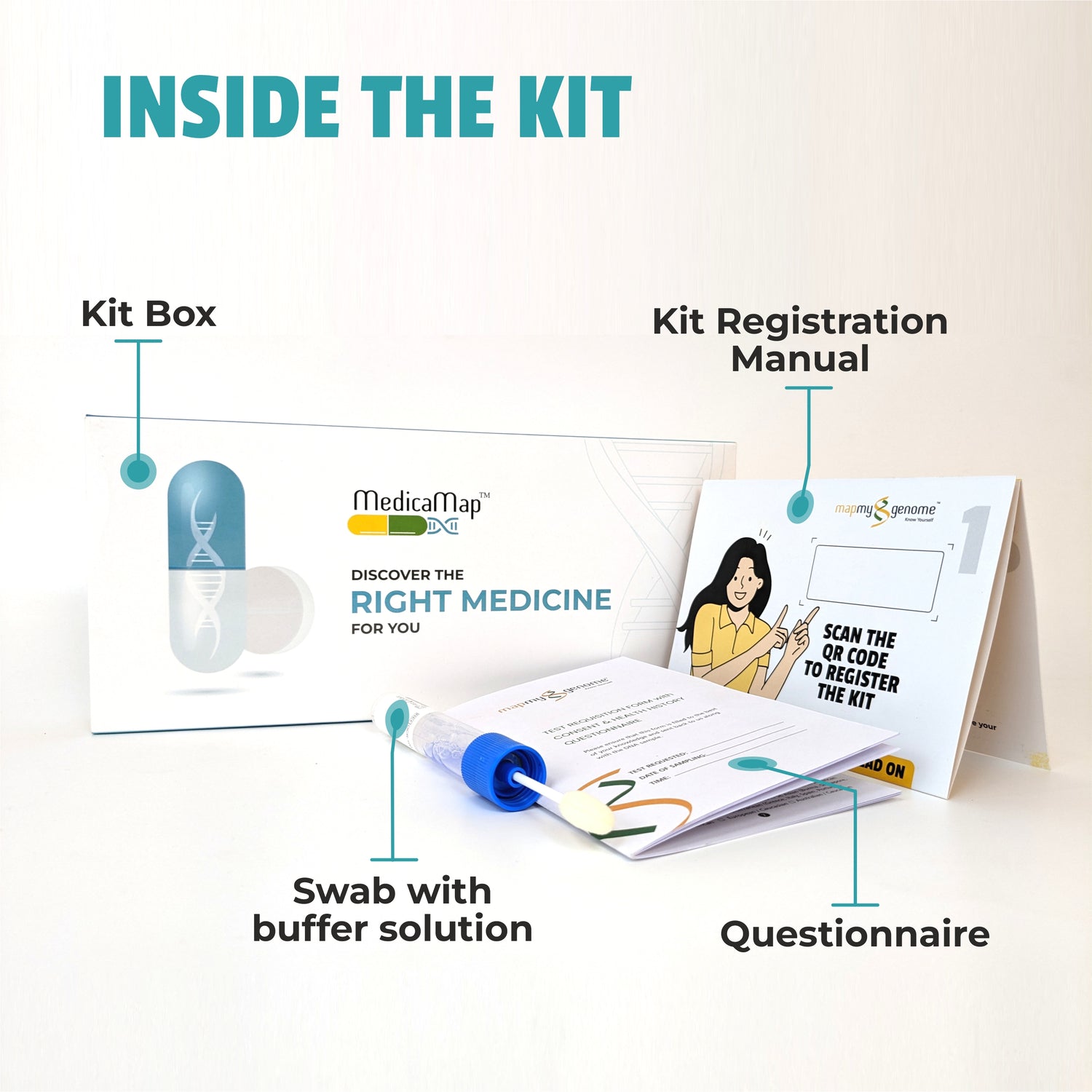
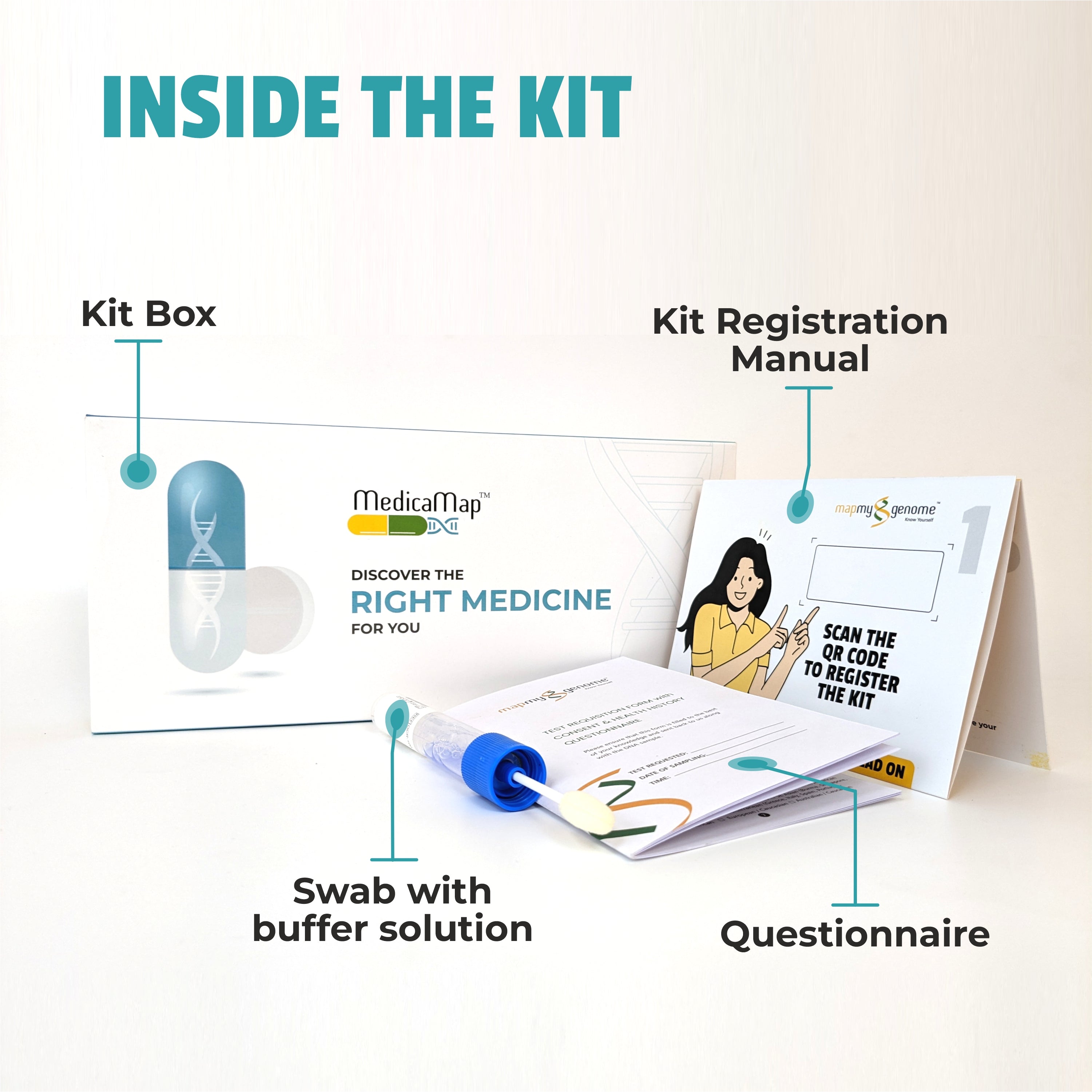
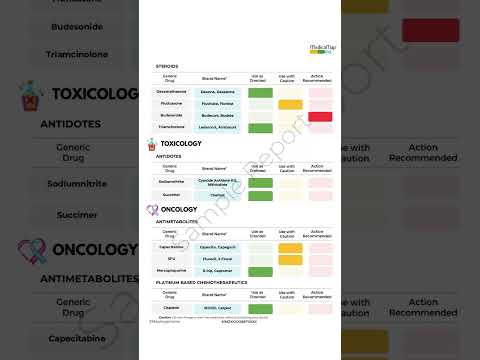
In recent years, healthcare has seen a remarkable shift towards personalized medicine—an approach that tailors treatment to the individual rather than the disease. At the heart of this transformation is pharmacogenomics, the study of how a person's genetic makeup influences their response to drugs. This cutting-edge field holds the potential to revolutionize the way medications are prescribed, making treatments more effective while minimizing adverse reactions.
What is Pharmacogenomics?
Pharmacogenomics combines the principles of pharmacology (the science of drugs) and genomics (the study of genes and their functions). Essentially, it aims to understand how variations in your genes affect how you metabolize medications. Different people may have the same condition, but the way they respond to a particular drug can differ vastly due to their genetic profile.
For instance, a medication that works perfectly for one person may have little effect on another, or it may cause severe side effects. Pharmacogenomics helps predict these responses, allowing healthcare providers to select the most effective and safe treatment options for each individual.
Why is Pharmacogenomics Important?
In traditional medicine, most treatments follow a “one-size-fits-all” approach. While effective for many, this method often overlooks the unique genetic factors that can influence drug efficacy and safety. Here’s why pharmacogenomics is crucial in personalized medicine:
-
Tailored Drug Selection: By analyzing a patient's DNA, healthcare providers can determine which medications will be most effective. This eliminates the guesswork often involved in prescribing treatments and ensures faster, more accurate results.
-
Reduced Adverse Drug Reactions: One of the most significant benefits of pharmacogenomics is its ability to predict and prevent harmful side effects. Patients with specific genetic variations may metabolize drugs too quickly or too slowly, leading to toxic levels in the bloodstream or insufficient therapeutic effects. Pharmacogenomics testing can identify such risks before treatment begins.
-
Improved Drug Dosing: Finding the right dosage is often a trial-and-error process. Pharmacogenomics allows healthcare providers to prescribe the optimal dose based on a person’s genetic profile, ensuring maximum efficacy with minimal side effects.
-
Personalized Treatment Plans: With the information from pharmacogenomics testing, healthcare providers can craft highly personalized treatment plans. This improves patient outcomes and reduces the time spent trying multiple medications.
How Does Pharmacogenomics Work?
Pharmacogenomic testing typically involves a simple saliva or blood sample. The DNA in the sample is analyzed for specific gene variants that are known to influence drug metabolism. The results of the test can help guide decisions about which medications are likely to work best for the patient and which may cause adverse effects.
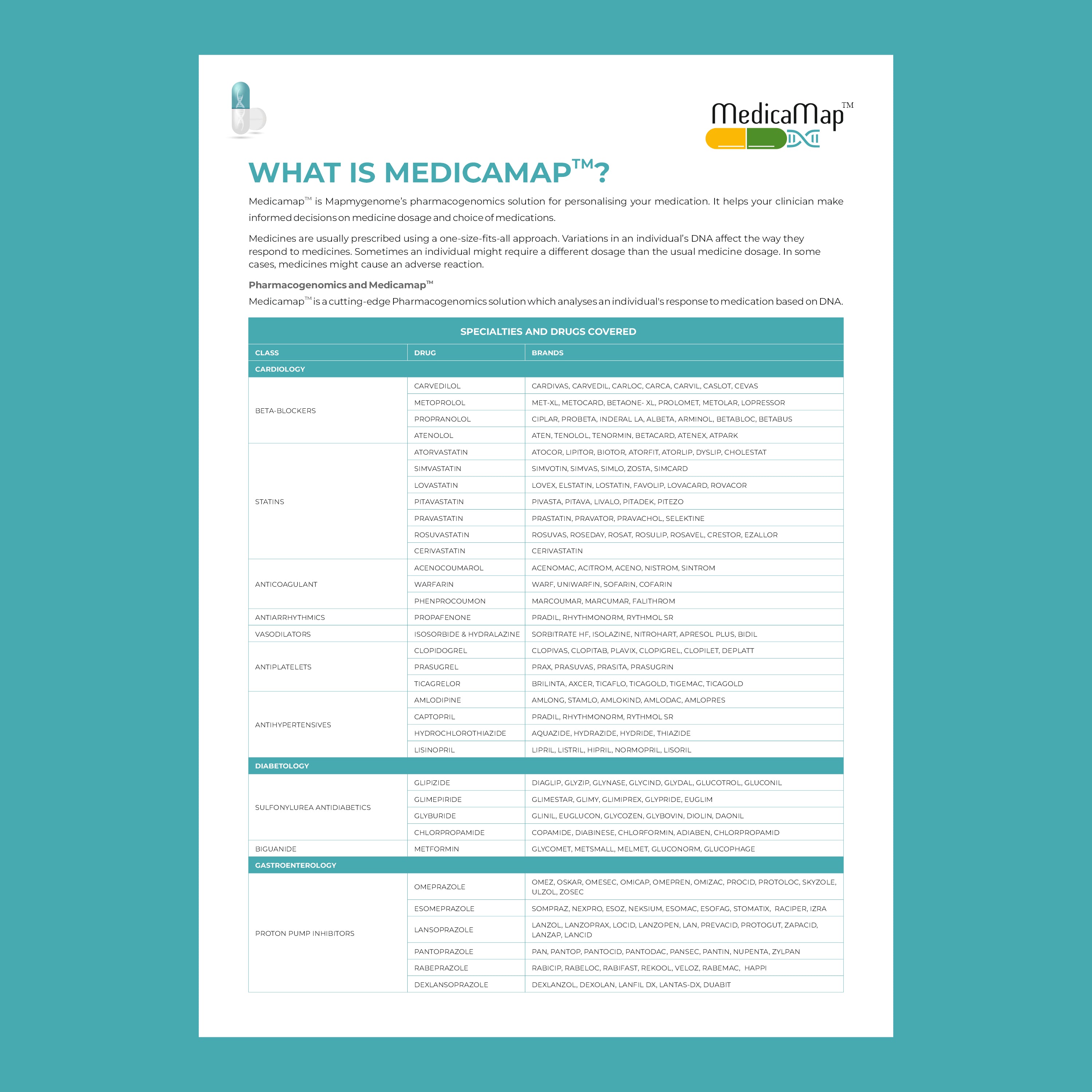
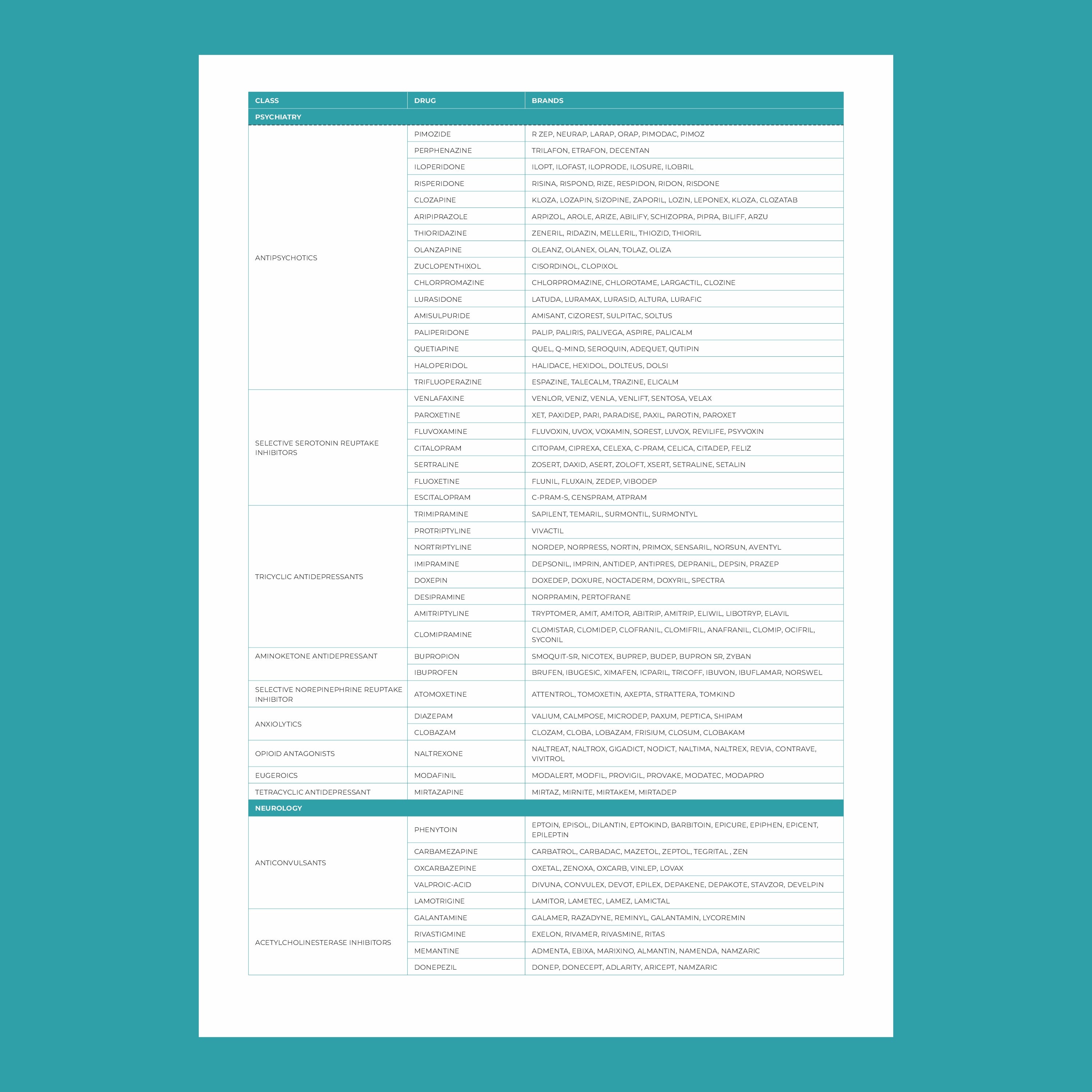
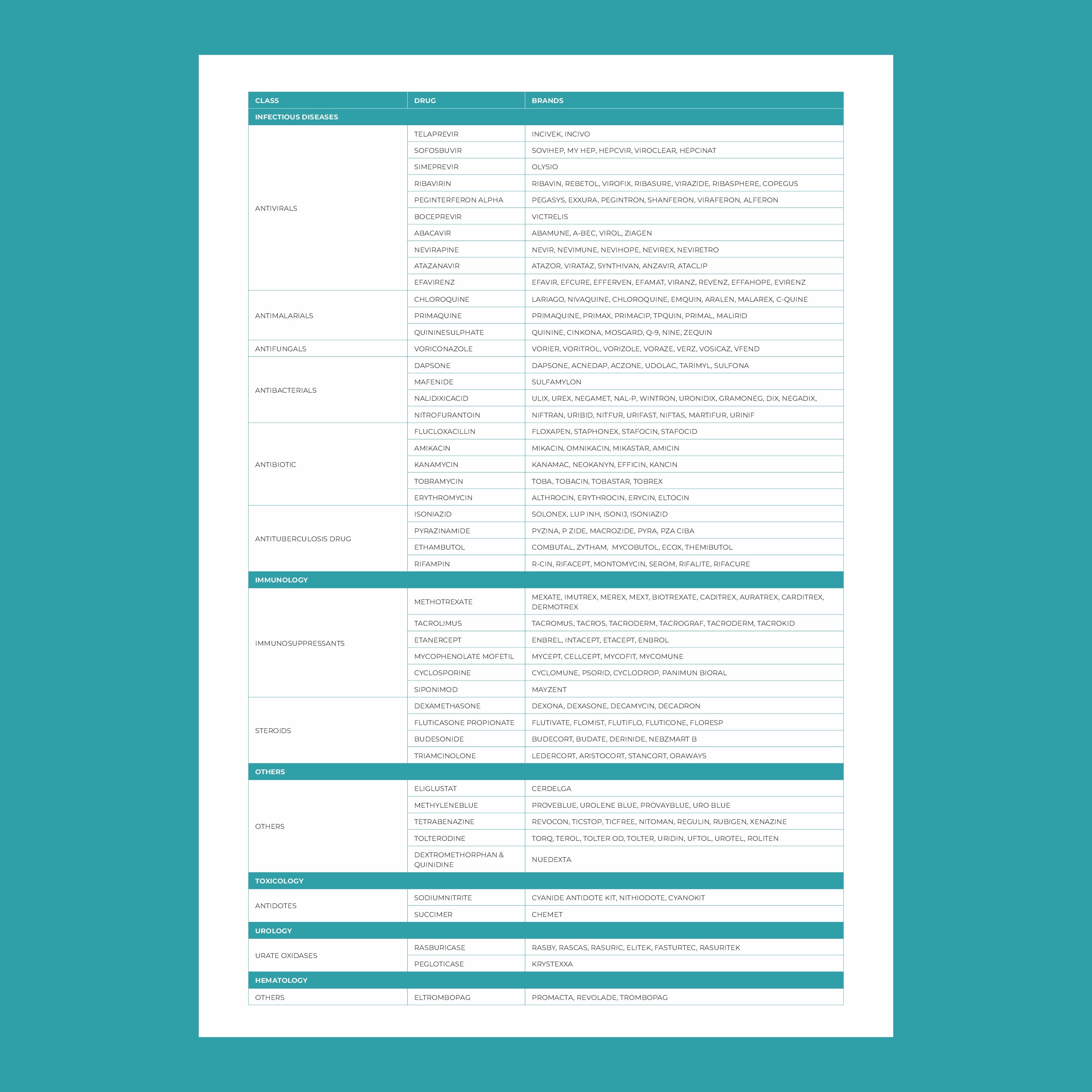
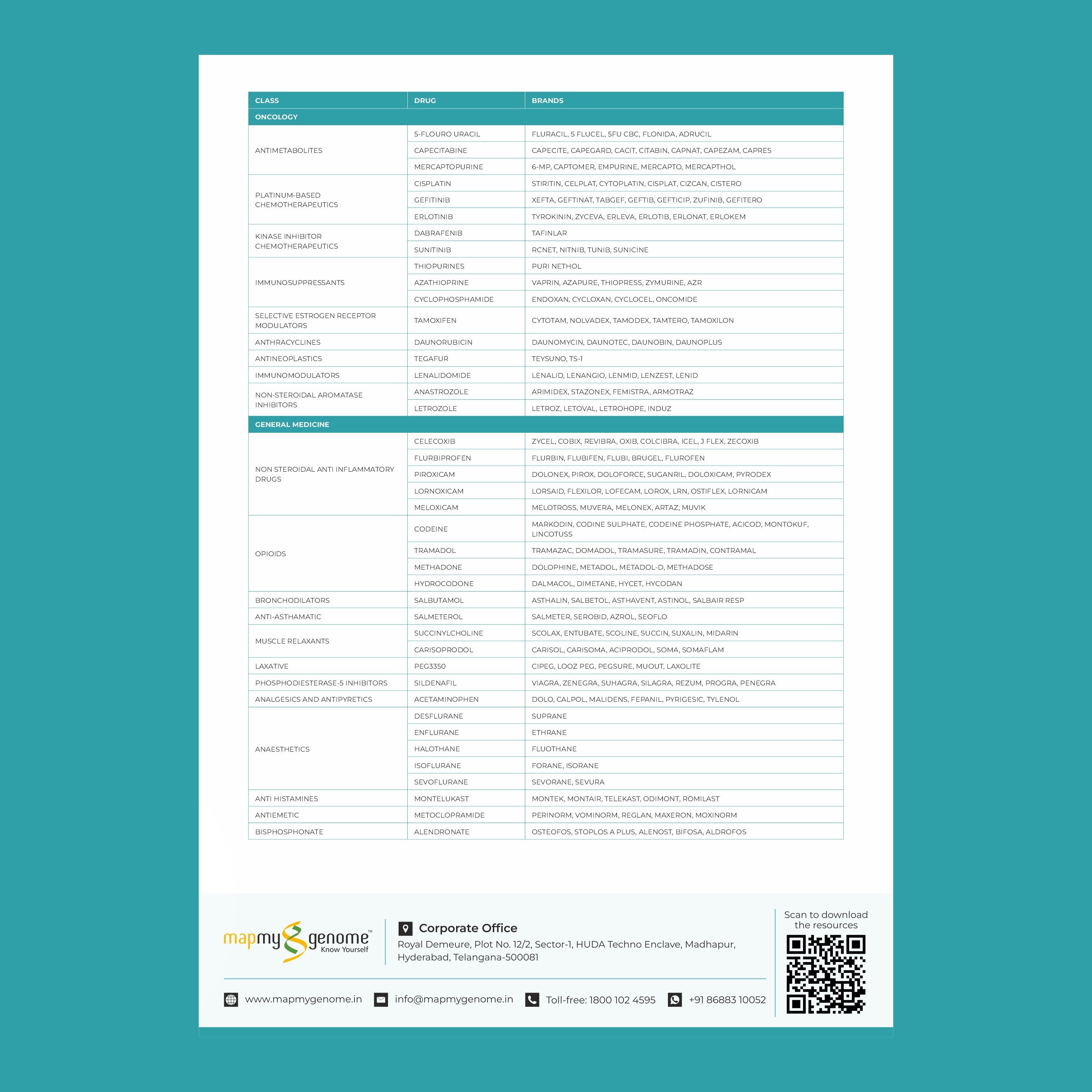
Key genes involved in pharmacogenomics include:
- CYP450 enzymes: These are responsible for metabolizing many drugs, including antidepressants, antipsychotics, and pain medications. Variants in these genes can affect how quickly or slowly a drug is metabolized.
- VKORC1: This gene affects the response to warfarin, a common blood thinner. People with certain variants of VKORC1 may require lower doses to avoid excessive bleeding.
- SLCO1B1: Variations in this gene can influence how statins (used to lower cholesterol) are processed in the body. Some individuals may be more prone to side effects like muscle pain.
Pharmacogenomics in Action: Case Studies
To better understand the impact of pharmacogenomics in personalized medicine, let’s look at a few examples:
1. Mental Health Medications
Many patients with depression or anxiety go through a long process of trial and error before finding the right antidepressant. Pharmacogenomics can streamline this process. By analyzing genes involved in drug metabolism, healthcare providers can predict which antidepressants are most likely to work and which may cause side effects. For example, variations in the CYP2C19 gene affect the processing of SSRIs (a common type of antidepressant), influencing the effectiveness and risk of side effects.
2. Cardiovascular Disease
Pharmacogenomics plays a key role in managing cardiovascular diseases. For example, the drug clopidogrel (used to prevent strokes and heart attacks) requires activation by enzymes in the liver. Individuals with certain genetic variants in the CYP2C19 gene may not activate the drug effectively, rendering it less effective. Genetic testing helps ensure patients receive the right antiplatelet therapy, reducing the risk of heart attacks or strokes.
3. Cancer Treatment
Cancer treatment has been a leading area for personalized medicine, especially pharmacogenomics. Drugs like tamoxifen (used for breast cancer) require activation by enzymes in the liver. Genetic variations in the CYP2D6 gene can affect how well the drug works. Pharmacogenomic testing helps oncologists determine the best course of treatment, improving outcomes for patients with cancer.
The Future of Pharmacogenomics
The field of pharmacogenomics is rapidly evolving, with new discoveries happening regularly. As genetic testing becomes more affordable and accessible, we can expect a significant shift towards pharmacogenomic-guided treatment in many areas of medicine.
Some of the exciting developments on the horizon include:
- Widespread Testing: Pharmacogenomics testing could become a routine part of medical care. Just like blood tests, genetic testing for drug metabolism may be done at the outset of treatment to guide therapy from the start.
- Artificial Intelligence (AI) Integration: With the power of AI, pharmacogenomics data can be processed and analyzed quickly, providing instant insights into the best treatment options for patients based on their genetic profiles.
- Expansion to Over-the-Counter Medications: Pharmacogenomics could extend beyond prescription drugs to over-the-counter medications. For instance, some people may have a genetic predisposition to side effects from common pain relievers like ibuprofen.
Challenges in Pharmacogenomics
While the potential of pharmacogenomics is immense, there are challenges to its widespread adoption:
-
Cost and Accessibility: Although the cost of genetic testing has decreased, it can still be a barrier for many. Widespread adoption will depend on making testing more affordable and ensuring insurance coverage.
-
Knowledge Gap: Many healthcare providers may not yet be fully trained in pharmacogenomics or how to interpret test results. Ongoing education will be essential for integrating pharmacogenomics into routine care.
-
Ethical Considerations: As with any genetic testing, there are concerns about privacy and how genetic information may be used. Clear regulations and protections will be crucial as pharmacogenomics becomes more common.
Conclusion
Pharmacogenomics is at the forefront of personalized medicine, offering a future where treatments are tailored to each individual’s genetic profile. By reducing adverse drug reactions, improving drug efficacy, and personalizing treatment plans, pharmacogenomics holds the promise of transforming healthcare. As the field continues to evolve, patients and healthcare providers alike will benefit from the precision and insight it offers.