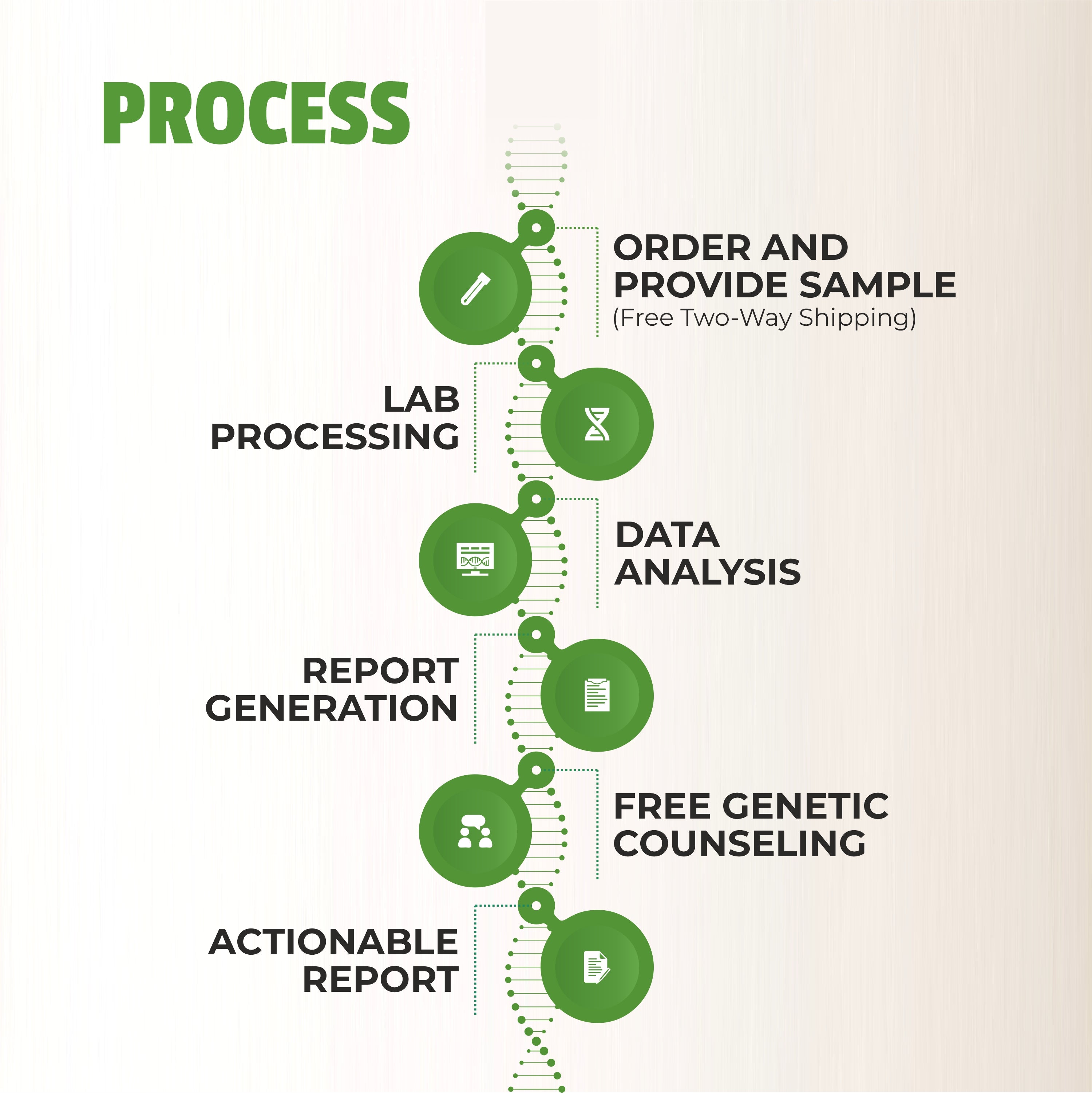
The field of clinical genomics is undergoing a profound transformation, driven by the rapid evolution of sequencing technologies. Some powerful tools have emerged as game-changers in this arena: Next-Generation Sequencing (NGS), cytogenetic testing, and Sanger sequencing. These technologies have enabled researchers and clinicians to unravel the intricacies of the human genome, offering invaluable insights into genetic diseases and personalized medicine. In this comprehensive blog, we will delve into the profound impact of these sequencing technologies on clinical genomics.
Next-Generation Sequencing (NGS)
Next-generation sequencing, often referred to as high-throughput sequencing, is a revolutionary technology that has reshaped our approach to genomics. It facilitates rapid and cost-effective analysis of DNA and RNA sequences, setting it apart from the laborious and costly Sanger sequencing method. NGS's capacity to process vast amounts of genetic data in parallel has made it a cornerstone of clinical genomics.
NGS has revolutionized the diagnosis of genetic disorders, enabling the sequencing of an individual's entire genome or just the exome (coding regions), which house most disease-associated variants. This comprehensive analysis simplifies the identification of genetic mutations underlying a broad spectrum of diseases, from rare genetic disorders to cancer. It has also ushered in the era of personalized medicine through pharmacogenomic studies, where genetic profiles inform treatment plans, maximizing efficacy while minimizing adverse effects. In the domain of oncology, NGS's ability to identify specific mutations, copy number variations, and fusion genes has deepened our understanding of cancer, guiding treatment decisions, including the use of targeted therapies and immunotherapies. Additionally, NGS has revolutionized all phases of reproductive health, enabling prospective parents to make better decisions.
There are several types of NGS approaches employed in clinical genomics:
- Whole Exome Sequencing (WES): WES focuses on sequencing only the protein-coding regions of the genome. It is a cost-effective strategy for identifying genetic variants associated with a wide range of diseases while avoiding the sequencing of non-coding regions.
- Whole Genome Sequencing (WGS): WGS involves the sequencing of the entire genome, including both coding and non-coding regions. While comprehensive, it generates a vast amount of data and is often reserved for complex cases or research studies.
- Targeted Sequencing: This approach hones in on specific regions of interest within the genome, such as genes associated with a particular disease or pathway. Targeted sequencing is efficient and cost-effective when the focus is on a defined set of genetic variants.
NGS brings speed and cost-effectiveness to genetic testing, making it accessible to a wider population. Its high sensitivity and accuracy have transformed the identification of disease-causing genetic variants, particularly for rare and complex disorders where pinpointing the genetic cause is challenging. Furthermore, NGS enables personalized medicine, tailoring treatment plans based on an individual's genetic makeup, optimizing therapeutic outcomes, and minimizing adverse reactions.
Cytogenetic Tests
Complementing NGS, cytogenetic testing is a vital component of clinical genomics. This technique involves the microscopic examination of chromosomes and their structural anomalies, such as translocations, deletions, or duplications, offering valuable insights at the chromosomal level.
Cytogenetic testing plays a crucial role in prenatal diagnosis, identifying chromosomal abnormalities in fetuses, including conditions like Down syndrome and Turner syndrome. In the field of oncology, it is instrumental in diagnosing and prognosticating various cancers, revealing specific chromosomal abnormalities associated with malignancies. Additionally, cytogenetic testing aids in investigating unexplained developmental delays or intellectual disabilities, shedding light on chromosomal abnormalities that may underlie these conditions.
Sanger Sequencing
Sanger sequencing, also known as chain-termination sequencing, represents a classical DNA sequencing method developed in the 1970s. While it has yielded much of its territory to NGS in large-scale sequencing projects, it remains relevant in clinical genomics.
Sanger sequencing is often used to validate specific genetic variants identified by NGS, ensuring their accuracy in a clinical context. It is a cost-effective approach for confirming the presence of known mutations when a specific genetic mutation is suspected. Sanger sequencing is highly accurate and reliable, making it well-suited for verifying individual genetic variants. It is also cost-effective when specific genomic regions need to be sequenced.
The Impact of Sequencing Technologies on Clinical Genomics
The impact of Next-Generation Sequencing (NGS), cytogenetic testing, and Sanger sequencing on clinical genomics is nothing short of revolutionary. These technologies have accelerated the pace of genetic discovery, transformed diagnostics, and personalized medicine, and paved the way for more effective treatments. By uncovering genetic insights at both the single-gene and chromosomal levels, these tools have equipped healthcare professionals with a comprehensive toolkit for understanding and addressing genetic diseases. They have also fostered collaboration across disciplines and driven advances in bioinformatics.
NGS, cytogenetic testing, and Sanger sequencing combinedly ushered in this new era of clinical genomics, empowering clinicians and researchers with unprecedented insights into the human genome. From diagnosing genetic disorders and enabling personalized medicine to identifying chromosomal abnormalities and confirming genetic variants, these technologies have revolutionized the way we approach healthcare. As technology continues to evolve and interdisciplinary collaboration flourishes, the future of clinical genomics holds the potential to revolutionize the lives of individuals affected by genetic diseases and shape the landscape of medicine for generations to come. The impact of these sequencing technologies will continue to deepen our understanding of genetics and drive advancements in clinical care.
Mapmygenome's diagnostic product portfolio helps in leveraging these advancements across different specialties like reproductive health, oncology, rare diseases, infectious diseases and many more. To learn more about these tests and other common screening tests prescribed by doctors like NIPT(Non-invasive prenatal testing) and CMA(Chromosomal Microarray).